The reactivity of compound Z with different halogens under appropriate conditions is given below :

The observed pattern of electrophilic substitution can be explained by
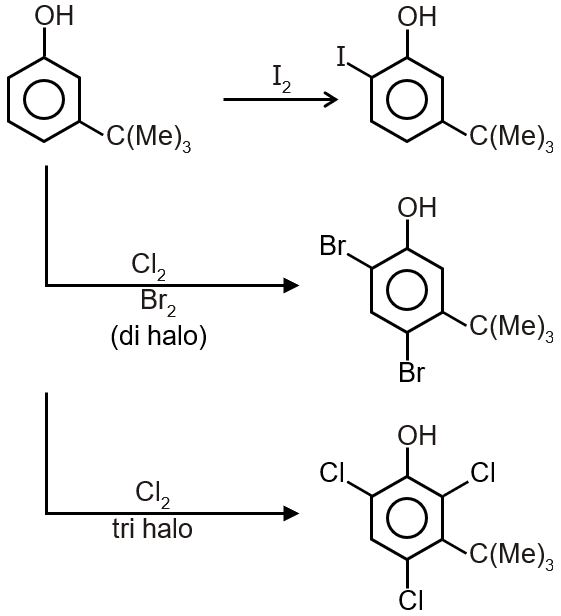
OH is strong activating +M group, so substitution of electrophile take place o/p to OH group and in substitution stearic hindrance of tert-butyl and Halogen also plays a role
The observed pattern of electrophilic substitution in compound Z (2-tert-butyl-4-methylphenol) can be explained by the steric effect of the tert-butyl group.
Let's analyze the structure and reactivity:
Step 1: Identify the directing effects
The phenolic -OH group is a strong ortho/para-directing group due to its electron-donating resonance effect. The tert-butyl group ((CH3)3C-) is also an ortho/para-directing group due to its electron-donating inductive effect. Both groups direct electrophiles to the ortho and para positions relative to themselves.
Step 2: Analyze the substitution pattern
The observed reactions show:
Position 6 is sterically less hindered compared to positions adjacent to the bulky tert-butyl group.
Step 3: Understand the steric effect
The tert-butyl group is very bulky (three methyl groups attached to a central carbon). This creates significant steric hindrance around the ortho positions to it (positions 3 and 5). Electrophiles avoid these positions due to steric repulsion, even though both electronic effects favor substitution at these positions.
Step 4: Compare with position 6
Position 6 is ortho to the OH group and para to the tert-butyl group. It is electronically activated by both groups and is sterically accessible because it is not adjacent to the bulky tert-butyl group. Thus, all electrophiles preferentially substitute at position 6.
Conclusion: The pattern is primarily governed by the steric effect of the tert-butyl group, which blocks substitution at positions 3 and 5, leaving position 6 as the most favorable site.
Electrophilic Aromatic Substitution: This is a fundamental reaction where an electrophile replaces a hydrogen atom on an aromatic ring. The rate and position of substitution are influenced by substituents already present on the ring.
Steric Effects: In organic chemistry, steric effects arise from the spatial arrangement of atoms. Bulky groups can hinder approach to reactive sites, overriding electronic preferences.
Directing Effects: Substituents on benzene rings influence the orientation of incoming electrophiles. Electron-donating groups (e.g., -OH, -CH3) are ortho/para-directing, while electron-withdrawing groups (e.g., -NO2, -COOH) are meta-directing.
No specific mathematical formulae apply here, but understanding these concepts is crucial: