Compound (A), C8H9Br, gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives an acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A).
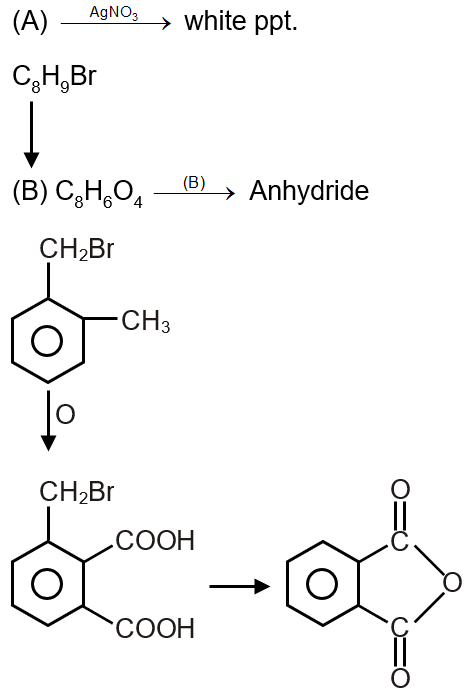
(B) Because it is giving precipitate so Br not attached to ring And anhydrisde form so position is ortho.
This problem involves identifying an organic compound (A) based on its molecular formula, reactions, and the properties of its oxidation product. Let's break it down step by step.
Step 1: Analyze the given information about compound (A)
Compound (A) has the molecular formula C8H9Br. It gives a white precipitate with alcoholic AgNO3 when warmed. This reaction is a test for alkyl halides that can form carbocations easily, typically tertiary, benzylic, or allylic halides. The white precipitate is AgBr, indicating that bromide is displaced, suggesting (A) is likely a bromide that undergoes SN1 reaction.
Step 2: Consider the oxidation product
Oxidation of (A) gives an acid (B) with formula C8H6O4. Notice that oxidation leads to loss of hydrogen atoms and gain of oxygen, consistent with side-chain oxidation in aromatic compounds. The product (B) is a dicarboxylic acid (since it has 4 oxygen atoms and forms an anhydride).
Step 3: Formation of anhydride
(B) easily forms an anhydride on heating. This is characteristic of dicarboxylic acids where the two carboxyl groups are adjacent to each other (phthalic acid type), allowing cyclic anhydride formation.
Step 4: Deduce the structure
Since (B) is C8H6O4 and forms an anhydride, it is likely ortho-phthalic acid. Oxidation of (A) gives this, so (A) must be an ortho-disubstituted benzene with a side chain that oxidizes to -COOH. The molecular formula of (A) is C8H9Br. Comparing with phthalic acid (C8H6O4), (A) has more hydrogens, indicating the presence of an alkyl group.
Possible side chain in (A): since oxidation gives dicarboxylic acid, it should have two alkyl groups ortho to each other. One is -Br and the other is -CH3 (or similar). Formula calculation: benzene ring C6H4 (disubstituted), plus -CH3 (C1H3) and -Br gives C7H7Br, but we need C8H9Br. So, it must be -CH2CH3 or similar. Actually, for ortho-xylene derivative: ortho-xylene is C8H10. Replacing one H with Br gives C8H9Br. Oxidation of ortho-xylene gives phthalic acid. So (A) is ortho-bromomethyl toluene? But careful: molecular formula C8H9Br suggests a benzene with two substituents: one Br and one C2H5? But that would be C8H9Br (benzene C6H5 + C2H4 + Br minus H = C8H9Br). However, oxidation of ethylbenzene gives benzoic acid, not dicarboxylic. So it must be disubstituted with groups on adjacent carbons.
Actually, for ortho-disubstituted with methyl and bromomethyl: that would be C6H4(CH3)(CH2Br) which is C8H9Br. Oxidation of methyl group gives -COOH, and oxidation of CH2Br would also give -COOH (since benzylic halide can oxidize). So (A) is 2-bromomethyl toluene (ortho).
Oxidation: both groups oxidize to -COOH, giving phthalic acid (C8H6O4). And it forms anhydride. Also, the bromine is benzylic, so it gives precipitate with AgNO3.
So compound (A) is , which is 2-(bromomethyl)-1-methylbenzene.
Looking at the options, the correct structure is the one with methyl and CH2Br adjacent on benzene ring.
Final Answer: The compound (A) is 2-(bromomethyl)-1-methylbenzene. Among the given options, it is the first structure shown (with CH3 and CH2Br ortho to each other).
1. Reactions of Alkyl Halides: Benzylic halides are reactive due to stability of benzylic carbocation, leading to easy substitution (e.g., with AgNO3).
2. Oxidation of Aromatic Compounds: Alkyl side chains on benzene oxidize to carboxyl groups with strong oxidizing agents like KMnO4.
3. Dicarboxylic Acids and Anhydride Formation: Ortho-dicarboxylic acids form cyclic anhydrides upon heating.
Molecular formula calculation: For disubstituted benzene, C6H4R1R2.
Oxidation reaction: