The major organic compound formed by the reaction of 1,1,1–trichloroethane with silver powder is:
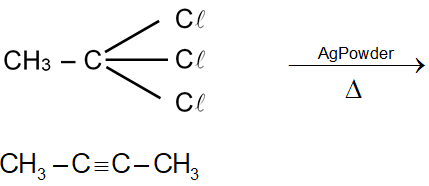
This question involves the reaction of 1,1,1-trichloroethane with silver powder, which is a classic method for the preparation of alkynes from vicinal or geminal dihalides. Let's break down the concept step by step.
The compound given is 1,1,1-trichloroethane. Its structure is: This is a geminal trihalide, meaning all three chlorine atoms are attached to the same carbon atom.
Silver powder (Ag) acts as a dehalogenating agent. It facilitates the removal of halogen atoms (Cl, Br, I) from the molecule. For geminal dihalides or trihalides, this reaction is specifically used to synthesize alkynes.
The reaction proceeds in two stages:
Stage 1: The geminal trihalide first loses two molecules of AgCl to form a carbene intermediate, which then rearranges to form a terminal alkyne.
The general reaction for a geminal dihalide is: (The carbene, R₁R₂C:, is unstable and isomerizes.)
For a geminal trihalide like 1,1,1-trichloroethane ( ), the reaction is similar. It acts as if it were a geminal dihalide with an extra halogen. The two chlorines on the same carbon are removed, leading to the formation of an alkyne.
The specific reaction for 1,1,1-trichloroethane is: The product is Propyne ( ), a terminal alkyne.
Now, let's look at the given options:
Our product, Propyne (), is not listed. However, Acetylene () is a terminal alkyne and is the simplest alkyne. The reaction of geminal dihalides (or trihalides acting as such) with silver powder is a standard laboratory method for preparing terminal alkynes. While the direct product from 1,1,1-trichloroethane is propyne, the question might be testing the general principle, and "Acetylene" is the only terminal alkyne option provided. It's important to note that the reaction is specific for alkyne formation, not alkenes.
Final Answer: The major organic product is a terminal alkyne. Among the options, Acetylene is the representative terminal alkyne, making it the best choice, even though the specific product from this reactant would be propyne.
Key Reaction: Dehalogenation of vicinal/geminal dihalides to form alkynes.
General Formula:
Geminal Dihalide to Alkyne:
Vicinal Dihalide to Alkyne:
Related Subtopic: This reaction falls under the preparation methods of alkynes (Alkyne).