The final solution contains
[Ag(NH3)2]+ and [Cu(NH3)4]2+
[Pb(NH3)4]2+ and [CoCl4]2–
[Al(NH3)4]3+ and [Cu(NH3)4]2+
[Ag(NH3)2]+ and [Ni(NH3)6]2+
A piston filled with 0.04 mol of an ideal gas expands reversibly from 50.0 mL to 375 mL at a constant temperature of 37.0°C. As it does so, it absorbs 208 J of heat. The values of q and w for the process will be: (R = 8.314 J / mol K) (ln 7.5 = 2.01)
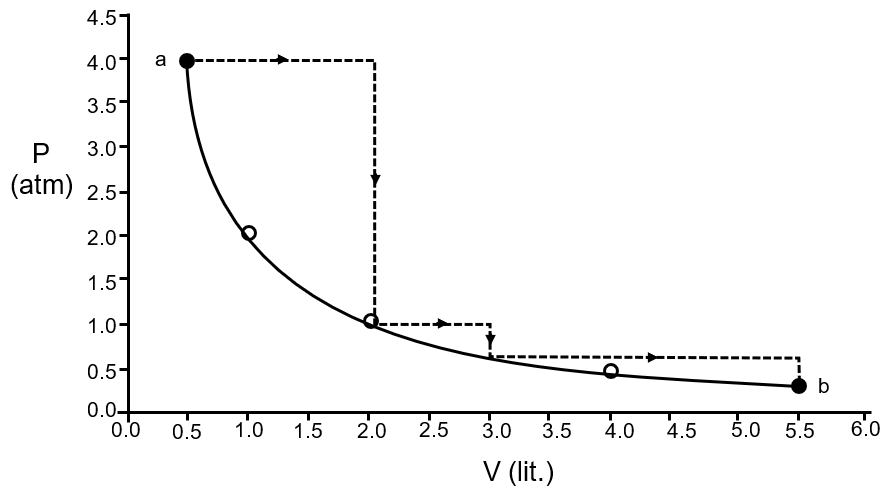
One mole of an ideal gas is taken from a to b along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is ws and that along the dotted line path is wd, then the integer closet to the ratio wd / ws is
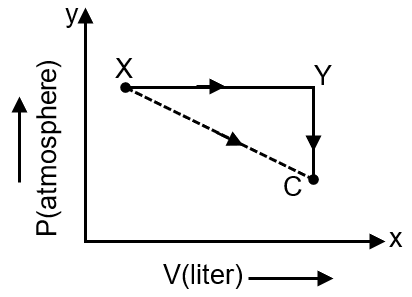
For an ideal gas, consider only P-V work in going from an initial state X to the final state Z. The final state Z can be reached by either of the two paths shown in the figure. Which of the following choice(s) is (are) correct ? [take ΔS as change in entropy and was work done]
Benzene and naphthalene form an ideal solution at room temperature. For this process, the true statement(s) is (are)
