The covalent alkaline earth metal halide (X = Cl, Br, I) is
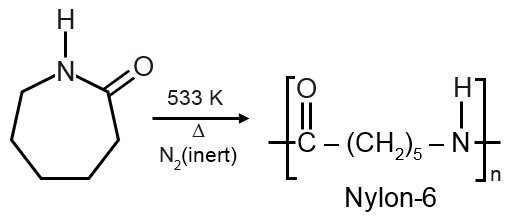
The direct monomer of Nylon-6 is caprolactum which polymerises to give Nylon-6 as follows:
The covalent alkaline earth metal halide is BeX2 (where X = Cl, Br, I).
Alkaline earth metals (Group 2 elements) typically form ionic compounds due to their low ionization energies. However, beryllium (Be) is an exception because of its small atomic size and high ionization energy. This results in a high polarizing power, which distorts the electron cloud of the anion (halide ion), leading to covalent character in its compounds.
The covalent nature can be explained using Fajans' rules:
This combination increases polarization, making BeX2 covalent. In contrast, MgX2, CaX2, and SrX2 are ionic due to larger cation sizes and lower polarizing power.
Related topics: Anomalous behavior of beryllium, Fajans' rules, and covalent character in ionic compounds.
Formulae: Polarizing power ∝ (charge / radius) of cation.