The correct structure of ethylenediaminetetraacetic acid (EDTA) is
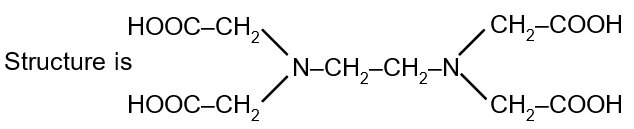
Ethylenediaminetetraacetic acid (EDTA) is a hexadentate ligand, meaning it has six donor atoms that can coordinate to a central metal ion. Its correct structure is derived from its name and its common function as a chelating agent.
Step 1: Break Down the Name
The name provides the molecular blueprint:
Step 2: Determine the Attachment Points
The four acetic acid groups are attached to the six donor atoms: the two nitrogen atoms of the amines and the four oxygen atoms of the carboxylate groups. A correct structure must have the four -CH2COOH groups bonded to the two nitrogen atoms.
Step 3: Analyze the Given Options
We must identify the structure where the central ethylene diamine backbone (H2N-CH2-CH2-NH2) has each nitrogen atom bonded to two acetic acid groups (-CH2COOH). The correct structure is represented by the third image, which shows this arrangement clearly.
Final Answer: The correct structure is the third option.
The general structural formula for EDTA is often written as:
Key Theory: EDTA is a classic example of a hexadentate ligand. Its two nitrogen and four oxygen atoms can simultaneously coordinate to a metal ion, wrapping around it and forming an extremely stable complex. This property makes it invaluable in analytical chemistry for titrations (complexometric titrations), in medicine as an antidote for metal poisoning, and in industry to sequester metal ions.