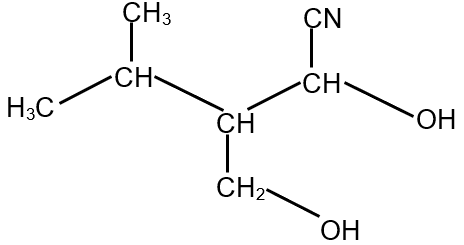
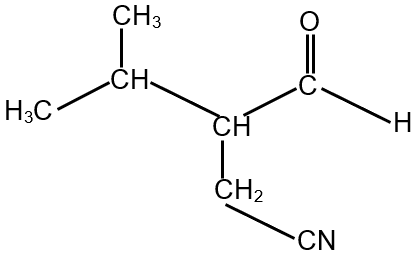
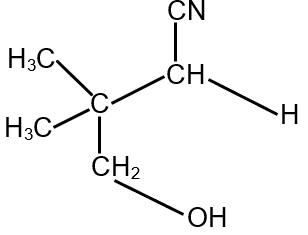
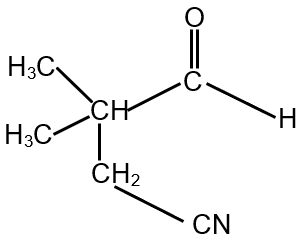
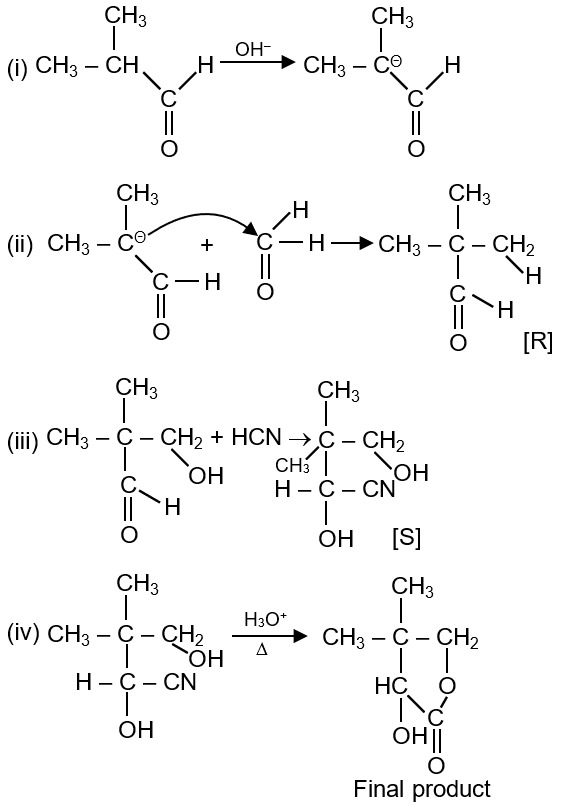
The compound S is
The entropy change involved in the isothermal reversible expansion of 2 moles of an ideal gas from a volume of 10 dm3 to a volume of 100 dm3 at 27°C is
Match the transformations in column I with appropriate options in column II
| Column-I | Column-II |
| (A) CO2(s) → CO2 (g) |
(p) phase transition |
| (B) CaCO3(s) → CaO(s) + CO2(g) |
(q) allotropic change |
| (C) 2H → H2(g) | (r) ΔH is positive |
| (D) P(white, solid) → P(red, solid) | (s) ΔS is positive |
| (t) ΔS is negative |
The reactions of Cl2 gas with cold-dilute and hot-concentrated NaOH in water give sodium salts of two (different) oxoacids of chlorine, P and Q, respectively. The Cl2 gas reacts with SO2 gas, in presence of charcoal, to give a product R. R reacts with while phosphorus to give a compound S. On hydrolysis, S gives an oxoacid of phosphorus, T.
