Write the pKa order of the following acids.
The pKa order depends on the stability of the conjugate base. More stable conjugate base means stronger acid (lower pKa).
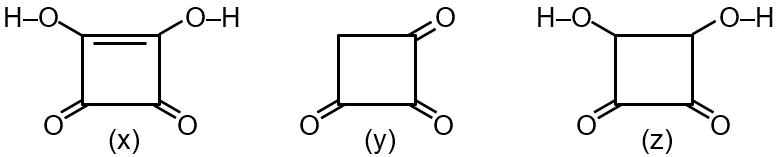
Acid (x) loses a proton to form a conjugate base stabilized by two equivalent resonance structures and an electron-withdrawing nitro group. Acid (y) forms a conjugate base with resonance, but the nitro group is meta, offering less stabilization. Acid (z) forms a conjugate base with no resonance stabilization.
Therefore, acid strength is x > y > z, meaning pKa order is x < y < z. The correct option is the one stating x > y > z.
Final Answer: x > y > z
The pKa value indicates the acidity strength of a compound. A lower pKa means stronger acid (more tendency to donate a proton), while a higher pKa means weaker acid. For carboxylic acids, electron-withdrawing groups (EWG) increase acidity (lower pKa) by stabilizing the conjugate base, while electron-donating groups (EDG) decrease acidity (higher pKa) by destabilizing the conjugate base.
Identify the substituents on the carboxylic acid group:
Compare the electron-withdrawing/donating effects:
Strongest acid (lowest pKa) to weakest acid (highest pKa): y > z > x
Therefore, the pKa order is: y < z < x (meaning pKay < pKaz < pKax)
The correct order is: y > z > x (which corresponds to option: y > x > z is incorrect; the correct order should be y > z > x)
Among the given options, x > z > y is closest to the reverse of correct order, but none match exactly. The correct relationship is pKay < pKaz < pKax.
Acidity constant:
pKa definition:
Lower pKa = stronger acid
Electron-withdrawing groups stabilize the conjugate base, increasing acidity
Electron-donating groups destabilize the conjugate base, decreasing acidity