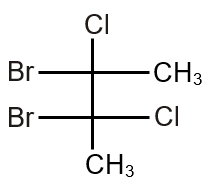
The total number(s) of stable conformers with non-zero dipole moment for the following compound is (are)
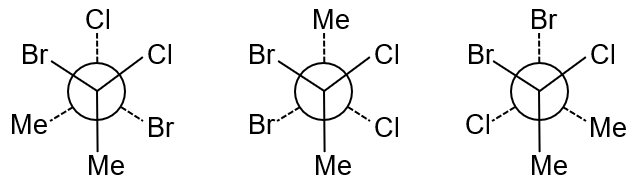
Stable conformers → Only Gauche form is considered
This question involves analyzing stable conformers of 1,2-dichloroethane (Cl-CH2-CH2-Cl) and identifying how many have a non-zero dipole moment. Conformers are different spatial arrangements of atoms resulting from rotation around single bonds.
Key Concepts:
1,2-dichloroethane can exist in several conformations due to rotation around the C-C bond. The main stable conformers are:
Dipole Moment Consideration:
The dipole moment depends on the vector sum of individual bond dipoles (C-Cl bonds have significant dipole moments). For the dipole moment to be non-zero, the molecular geometry must not have perfect symmetry canceling out the dipoles.
Analysis of Stable Conformers:
Only staggered conformers are considered stable (minimized torsional strain):
There are two equivalent gauche conformers (clockwise and anticlockwise rotation), but both have identical non-zero dipole moments. However, the question asks for the number of stable conformers with non-zero dipole moment, not the distinct values. Since both gauche conformers are stable and have non-zero dipole moment, the answer is 2.
Final Answer: 2 stable conformers (both gauche) have non-zero dipole moment.
Conformational Isomerism: Study of different arrangements of atoms in a molecule that can be interconverted by rotation around single bonds.
Dipole Moment (μ): A measure of molecular polarity, calculated as the vector sum of individual bond dipoles. μ = q × d, where q is charge magnitude and d is distance.
Stability Order: Staggered (anti and gauche) > Eclipsed. Anti is most stable due to minimized steric and electronic repulsions, but gauche has higher energy due to chlorine clash.