The correct order regarding bond length :
(i) N2H2 < N2H4 (N – N Bond length)
(ii) NO > NO+ (N – O Bond length)
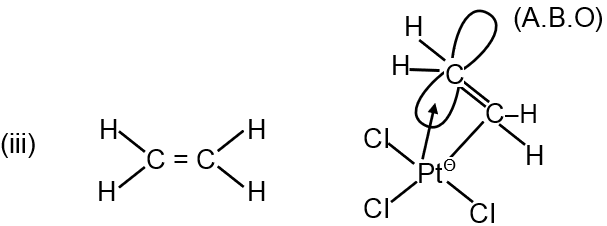
(iii) C2H4 > K[PtCl3(C2H4)] (C – C Bond length)
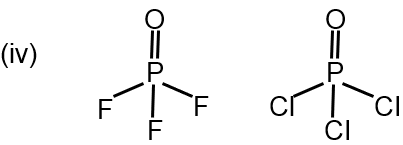
(iv) POF3 > POCl3 (P – O Bond length)
(i) N2H2 < N2H4 BL double bond < BLsingle bond
(ii) NO > NO+
B.O. → NO+ > NO
BO e– accepted in anti bonding orbital.
BL
C2H4 < K [PtCl3(C2H5)] [C–C (B.L.)]
→ s back bonding is comparable so we consider % s character.
EN → F > Cl
% s character → P–F < P–Cl
% s character of P=O , POF3 > POCl3
% s α B.L. ⇒ POF3 < POCl3
Bond length is the average distance between the nuclei of two bonded atoms. It is influenced by bond order (higher order = shorter bond), atomic size, and electron distribution (resonance, back-bonding). Let's analyze each case step by step.
N₂H₂ (diazene) has a N=N double bond (bond order = 2). N₂H₄ (hydrazine) has a N–N single bond (bond order = 1). Since bond length is inversely proportional to bond order, the double bond in N₂H₂ is shorter than the single bond in N₂H₄. Therefore, the statement N₂H₂ < N₂H₄ is correct.
Nitric oxide (NO) has a bond order of 2.5. Its molecular orbital configuration is: . The nitrosyl ion (NO⁺) loses an antibonding electron, resulting in a configuration of , giving it a bond order of 3. A higher bond order means a shorter bond. Therefore, the bond in NO⁺ is shorter than in NO, making the statement NO > NO⁺ correct.
In free ethene (C₂H₄), the C=C bond is a double bond. In Zeise's salt, K[PtCl₃(C₂H₄)], ethene acts as a π-acid ligand. The metal atom (Pt²⁺) donates electron density back into the π* antibonding orbital of ethene. This back-donation weakens the C=C bond, reducing its bond order and increasing its length. Therefore, the C–C bond is longer in the complex than in free ethene. The statement C₂H₄ > K[PtCl₃(C₂H₄)] is correct.
This involves comparing the P–O bond in two oxyhalides. The key concept is π-bonding or back-bonding. Oxygen can donate a lone pair to the vacant d-orbital of phosphorus, forming a dπ-pπ bond. This shortens and strengthens the P–O bond. The extent of this back-bonding is greater when the halogens attached to phosphorus are less electronegative. Chlorine (Cl) is less electronegative than Fluorine (F). Therefore, back-bonding is more significant in POCl₃ than in POF₃. This results in a shorter P–O bond in POCl₃. The statement claims POF₃ has a longer bond, so POF₃ > POCl₃ is correct.
All four statements (i), (ii), (iii), and (iv) are correct. However, looking at the provided options, the one that includes all correct statements is: (i), (ii) & (iv). Note that option (i), (ii) & (iv) is correct, and statement (iii) is also correct but is not included in this particular option. Based on the options given, (i), (ii) & (iv) is the best choice as it contains only correct statements.
Bond length is a fundamental property that helps predict molecular geometry and reactivity. Factors decreasing bond length include:
- Increase in bond order (single < double < triple).
- Increase in s-character of the bond (sp³ > sp² > sp).
- Presence of π-backdonation (shortens the bond involved).
- Higher electronegativity difference can sometimes lead to shorter bonds due to increased ionic character.